Using argon or nitrogen for wine preservation revolves around their ability to displace oxygen, which is the primary cause of wine oxidation and spoilage. Both Argon and Nitrogen are used as wine preservation methods, but it is important to understand why one of the other may (or may not) work to keep your wine tasting fresh.
Here’s how each gas fares in the context of wine preservation:
Argon as a Wine Preservation Method
- Density: Argon is denser than air, allowing it to effectively blanket the wine by sitting on the surface, minimizing oxygen’s contact with the wine. This characteristic makes it particularly effective at preserving wine, especially in the context of an open bottle. More about this below...
- Inertness: As an inert gas, argon does not react with the wine, ensuring that the taste and aroma of the wine remain unaltered.
- Efficiency: Because of its density and inertness, argon is considered highly efficient for preserving wine, even in open bottles. It keeps wine tasting fresh because it reliably sinks below the oxygen to create the neutral barrier between the surface of the wine and the oxygen remaining inside the bottle.
Nitrogen as a Wine Preservation Method
- Density: Nitrogen is much lighter than argon and is also slightly lighter than oxygen. While it can displace oxygen if you can ensure all oxygen is replaced with nitrogen, this happens only in "closed" systems where oxygen is no longer present at all. In any open system, where oxygen remains present - like the inside of an open bottle of wine - nitrogen won't form as reliable or effective a barrier as the heavier argon does.
- Inertness: Nitrogen is also an inert gas and does not react with wine, maintaining the wine's integrity without altering its flavor or aroma.
- Cost-effectiveness: Nitrogen is abundant and commonly used in the food and beverage industry, making it a cost-effective option for wine preservation. It can still be effective in reducing oxidation, but again requires more technology to ensure all oxygen is vacated from the wine dispensing system.
The Science of Argon and Nitrogen
For a gas to be effective for preserving wine, it needs to be:
- Non-reactive with the wine
- Able to be inserted into the bottle
- Non-toxic, food safe
- Economically available
Both argon and nitrogen meet all of these criteria. But, how you deliver the gas and how you ensure it is effective in the system used to dispense the wine is where argon and nitrogen differ as effective wine preservation methods.
A key characteristic that drives delivery and management of the preservation gas is how "heavy" the gas is. Let's take a trip back to High School chemistry class, shall we..... In particular, the Periodic Table.
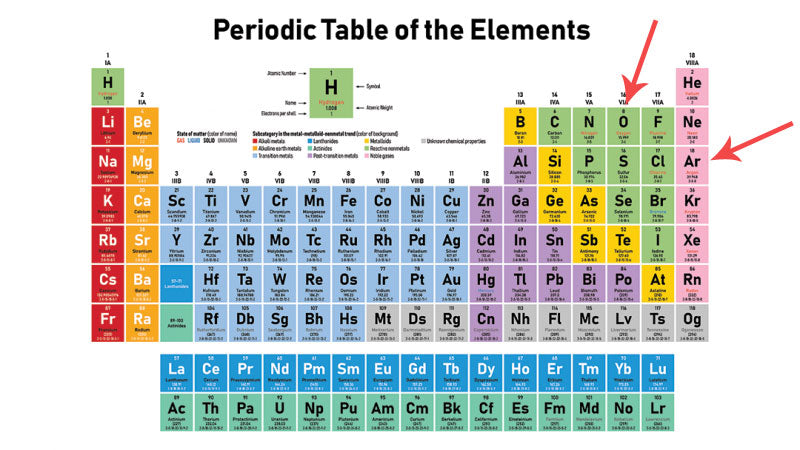
Atomic Mass is the measurement of how heavy an element is. In the context of wine preservation, atomic mass determines if the gas in question is heavier, or lighter, than oxygen.
The three gases involved here - argon, oxygen, and nitrogen, have the following atomic mass:
- Ar 39.948 - much heavier than oxygen
- O 15.999 - the reference point
- N 14.007 - lighter than oxygen
Check out the periodic table below, with oxygen and argon pointed out. Nitrogen (the letter N) is just to the left (or lighter) than oxygen.

If the gas is heavier than oxygen, than the gas will naturally descend inside the bottle of wine until it settles on top of the surface of the wine. If the gas is lighter than oxygen - even by a little bit - the gas will readily mix with the oxygen, or even ascend inside the bottle. This will leave a layer of oxygen against the surface of the wine, enabling oxidation to occur.
Here's the situation we want - the gas settling down on top of the surface of the wine, creating the protective barrier. This image shows VineyardFresh, but any 100% argon gas introduced into the wine bottle will do this.

Practical Considerations
- Usage: Both gases are used in wine preservation systems, with some systems specifically designed for one gas type. Nitrogen is reliably effective in closed loop systems like cabinets and/or large commercial tank installations.
- For home use, and use in fast paced commercial settings where installed cabinets or closed systems are cost prohibitive, argon delivered via a recyclable aluminum canister is the easiest and most cost effective solution
- Cost: The cost will vary depending on the preservation system in use. The cost of the two gases may not vary significantly, but the cost of the additional systems needed in the case of Nitrogen can be very significant.
So What?
All of this science and practical considerations lead us to the following conclusion:
Because argon is a noble gas, food safe, can be delivered into an open bottle of wine easily and inexpensively, and is heavier than oxygen - it is the preferred gas to use to preserve open bottles of wine

What Wine Preservers Use Argon?
- Most cabinets, including Wine Stations, which pipe argon into the bottle(s)
- Coravin, which introduces a burst of argon into the bottle after the pour
- Canisters, including VineyardFresh, Silvadore, and ArT
Pick up a can of VineyardFresh or Silvadore (our sister brand), and take advantage of using argon to preserve your wine. Enjoy the freedom to drink what you want, when you want, while supporting your healthier lifestyle of wellness and sustainability.